Industry Insights
Home > News > Industry Insight > Wecare's BLa80: China's Sole Infant Whitelist & Dual FDA GRAS Probiotic Pioneer Drives Industry to New Standards
Wecare's BLa80: China's Sole Infant Whitelist & Dual FDA GRAS Probiotic Pioneer Drives Industry to New Standards

Beijing, China – July 30, 2025 – Wecare Probiotics announced the successful conclusion of its pivotal seminar, "Clinically Validated + Potency Guaranteed + Officially Registered: Driving Probiotic Products Back to Fundamentals." The event specifically highlighted the groundbreaking inclusion into the Infant Food Whitelist of the BLa80 strain.
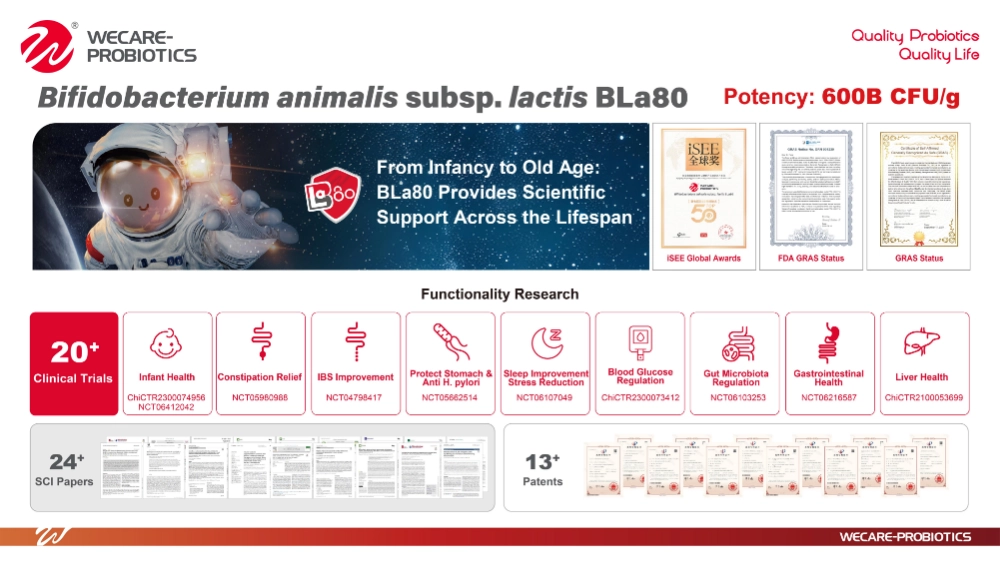
BLa80 now stands as the only probiotic strain in mainland China to be included on the National Health Commission's Infant Food Strain Whitelist, while also holding both FDA GRAS and self-affirmed GRAS status. Key aspects of the seminar on BLa80’s inclusion include:
Segmented Clinical Study Design: Trials are meticulously stratified across infant age groups (0-6 months, 7-12 months, 13-36 months) .
Multi-Center Collaboration & Rigorous Sample Sizes: Adherence to recommendations for multiple research centers and substantial sample sizes (≥400 subjects, including ≥1,100 infants in BLa80's case)
Global Standardized Design: Alignment with international guidelines such as GCP and CONSORT for double-blind, randomized controlled trials

BLa80 exemplifies Wecare's dedication, backed by over 20 clinical studies involving more than 2,500 subjects, including 1,100 infants, covering the entire infant growth cycle.








 Leave a Message
Leave a Message Email
Email Linkedin
Linkedin