Industry Insights
Development and Application of an Anti-Allergic Probiotic Agent

Abstract: This study introduces a novel probiotic agent with significant anti-allergic properties. The agent comprises two strains: Bifidobacterium animalis subsp. lactis BLa36 and Lacticaseibacillus casei LC89. The synergistic interaction between these strains enhances their efficacy in alleviating allergic reactions, providing a new strategy for preventing and treating allergic diseases. Due to the probiotic nature of both strains, the agent is safe and unlikely to induce resistance.
Introduction: Allergic diseases, including asthma, allergic rhinitis, food allergies, and atopic dermatitis, are influenced by genetic, environmental, lifestyle, and microbiome factors. The imbalance of gut microbiota is a critical factor in the development of allergic diseases. The gut, the largest immune organ in the body, is closely linked to host health through its microbiota, which affects gut barrier function and immune response. Probiotics, as key components of gut microbiota, can maintain microbial balance, enhance gut barrier function, and regulate the immune system, thus preventing and treating allergic diseases.
Background: Despite the recognized potential of probiotics in treating allergic diseases, current market products have limited efficacy. This is mainly due to the varying effects of different probiotic strains on allergies and the unclear mechanisms behind their anti-allergic actions. Therefore, developing a probiotic agent with a clear mechanism and high efficacy is essential for improving treatment outcomes and patients' quality of life.
Objectives: The primary goal of this research is to develop an anti-allergic probiotic agent that effectively regulates the immune system, enhances gut barrier function, and restores microbial balance. This agent should also be safe, stable, and suitable for clinical applications.
Methods:
1. Strain Selection: The probiotic agent includes Bifidobacterium animalis subsp. lactis BLa36 (CGMCC No. 24029) and Lacticaseibacillus casei LC89 (CGMCC No. 15409).
2. Synergistic Formulation: These strains are combined to leverage their potential interaction, enhancing their anti-allergic effects.
3. Probiotic Properties:

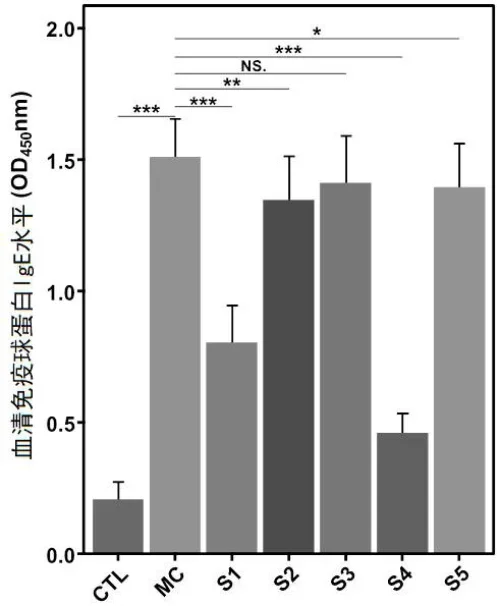
o Decrease in specific IgE levels in allergic disease models.
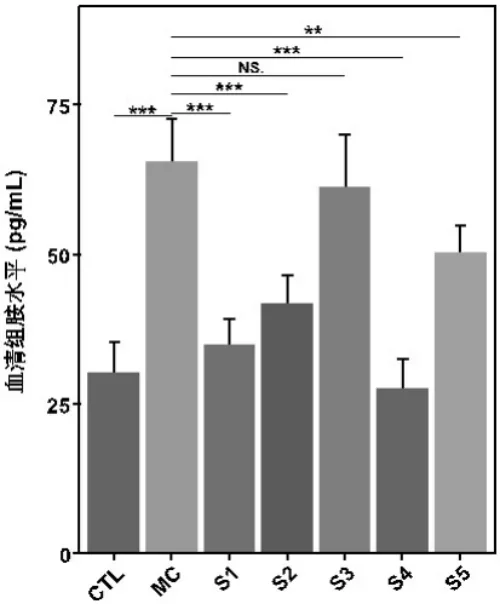
o Reduction of histamine release from mast cells.
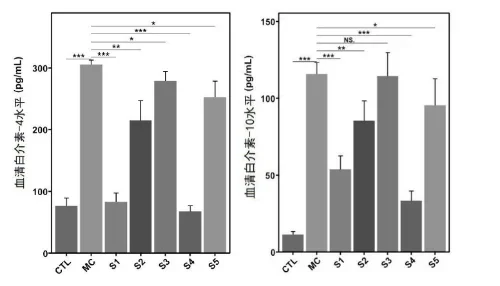
o Regulation of Th1/Th2 balance, promoting Th1 and suppressing Th2 cytokines.
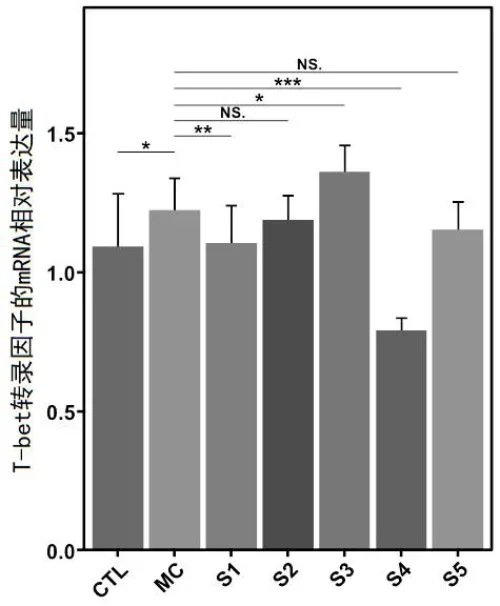
o Modulation of allergy-related gene expression in spleen T cells.
Results: The combination of BLa36 and LC89 strains significantly improved outcomes in allergic models compared to individual strains. The synergistic formulation effectively reduced IgE levels, decreased histamine release, and modulated immune responses, offering a new strategy for allergy prevention and treatment.
Applications: The developed probiotic agent can be formulated into various forms, including lyophilized powder, capsules, tablets, or granules. It can be used in products aimed at preventing, alleviating, or treating allergic reactions or diseases such as asthma, allergic rhinitis, food allergies, pollen allergies, and atopic dermatitis.
Conclusion: This study presents a novel anti-allergic probiotic agent comprising Bifidobacterium animalis subsp. lactis BLa36 and Lacticaseibacillus casei LC89. The synergistic interaction between these strains enhances their anti-allergic efficacy, providing a promising new approach for the prevention and treatment of allergic diseases. The safety and stability of the probiotic agent make it suitable for clinical applications, potentially improving the quality of life for individuals with allergies.
Keywords: Probiotic, Allergy, Bifidobacterium animalis subsp. lactis, Lacticaseibacillus casei, IgE, Histamine, Th1/Th2 balance, Immune modulation






 Leave a Message
Leave a Message Email
Email Linkedin
Linkedin